Abstract
Introduction
Acute myeloid leukemia (AML) with "TP53 mutation, chromosomal aneuploidy or both" has recently been described as a distinct AML subgroup exhibiting an exceedingly adverse prognosis. Therefore, testing for TP53 mutations has become an essential part of the European LeukemiaNet 2017 recommendations for risk classification. However, the incidence and pathogenic role of subclonal TP53 mutations defined by a variant allele frequency (VAF) <20% has not been addressed in this disorder yet.
Methods
We evaluated the prognostic value of TP53 VAFs in a cohort of 1537 patients with newly diagnosed AML, prospectively treated within 3 trials of the "German-Austrian AML Study Group". Mutation testing was performed by targeted deep sequencing and patients with TP53 mutations were categorized into 3 groups defined by VAFs <20%, 20%-40% and >40%, respectively.
Results
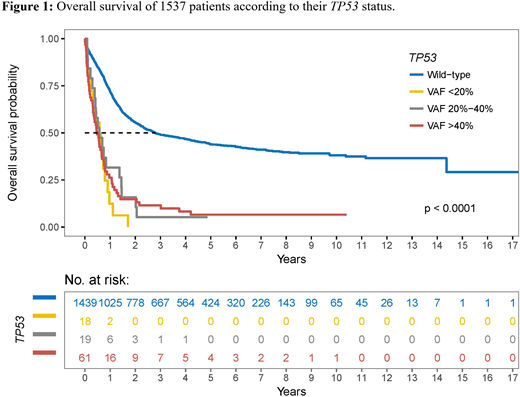
A total of 108 TP53 mutations were identified in 98 patients (6.4%). Amongst those, 61 patients showed a VAF >40%, 19 a VAF between 20% and 40% and 18 a VAF <20%. In either group, the majority of mutations were missense substitutions (VAF >40%, 49/61 [80%]; 20%-40%, 17/19 [89%]; <20%, 17/18 [94%]) and were located in the DNA binding domain of the gene (VAF >40%, 49/61 [80%]; 20%-40%, 16/19 [84%]; <20%, 16/18 [89%]). Compared to AML with clonal TP53 mutations, those with subclonal ones showed significantly fewer complex karyotypes and chromosomal losses. The number of cooperating driver mutations was low with 0-5 per case and did not differ between the 3 TP53 mutated groups. The complete remission (CR) rate was significantly inferior in the TP53 mutated cohort (48.0% versus 84.9% for TP53 wild-type patients, P <0.001) but equally distributed among the VAF-based subgroups (TP53 VAF >40%, 42.6%; 20%-40%, 57.9%; <20%, 55.6%; P=0.424). The estimated median overall survival (OS) for all 1537 AML patients was 28.1 months (95% CI, 24.3-33.5) but differed substantially between TP53 wild-type and TP53 mutated patients (33.6 months [95%CI, 28.4-45.0] versus 6.5 months [95%CI, 5.0-8.2]). As depicted in Figure 1, OS rates were comparably low in all TP53 mutated subgroups (TP53 VAF >40%, 5.8 months; 20%-40%, 6.9 months; <20%, 6.9 months).The median estimated event-free survival (EFS) for all 1537 AML patients was 15.0 months (95% CI, 13.6-16.5) with that for TP53 wild-type patients being 16.5 months (95% CI, 15.0-18.2) and for TP53 mutated patients 5.7 months (95% CI, 4.3-7.4). Median EFS rates were comparably low in all TP53 mutated subgroups (TP53 VAF >40%, 5.2 months; 20%-40%, 6.9 months; <20%, 6.5 months). In Cox regression analyses adjusting for age, white blood cell count, cytogenetic risk and type of AML, the adverse prognostic effect of TP53 mutations remained significant, with subclonal mutations showing the strongest impact (hazard ratio, 3.71 [95% CI, 2.11-6.51] for OS).
Conclusion
In our study on a large cohort of AML patients, TP53 mutated subclones defined by VAF <20% account for ~18% of all TP53 mutated AML cases. We here provide evidence that subclonal TP53 mutations are associated with a comparable negative impact on prognosis as clonal TP53 mutations. These findings may have implications for TP53 screening methods and for future risk stratification in AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal